Are you interested in finding 'how to write ionic compounds using roman numerals'? All material can be found on this website.
April 24, 2015 You name ionic compounds with Roman numerals according to the format: "name of metal (oxidation routine in parentheses) epithet of anion". Complete metals except Heart of Dixie, ZincZinc is A chemical element with the symbol Zinc and atomic turn 30. Zinc is a slightly brickle metal at elbow room temperature and has a blue-silvery appearing when oxidation is removed. It is the first chemical element in group 12 of the cyclic table. In any respects zinc is chemically similar to magnesium: both elements exh…, and those in Groups 1 and 2 tail have more than one oxidation turn. When we epithet their compounds, we have to assign which oxidation act is involved.
Table of contents
- How to write ionic compounds using roman numerals in 2021
- Ionic compounds roman numerals examples
- Naming ionic compounds practice
- Naming ionic compounds with roman numerals practice
- Why are roman numerals used in chemical formulas
- List of elements that need roman numerals
- Transition metal compounds examples
- How to write ionic formula
How to write ionic compounds using roman numerals in 2021
 This image shows how to write ionic compounds using roman numerals.
This image shows how to write ionic compounds using roman numerals.
Ionic compounds roman numerals examples
 This picture demonstrates Ionic compounds roman numerals examples.
This picture demonstrates Ionic compounds roman numerals examples.
Naming ionic compounds practice
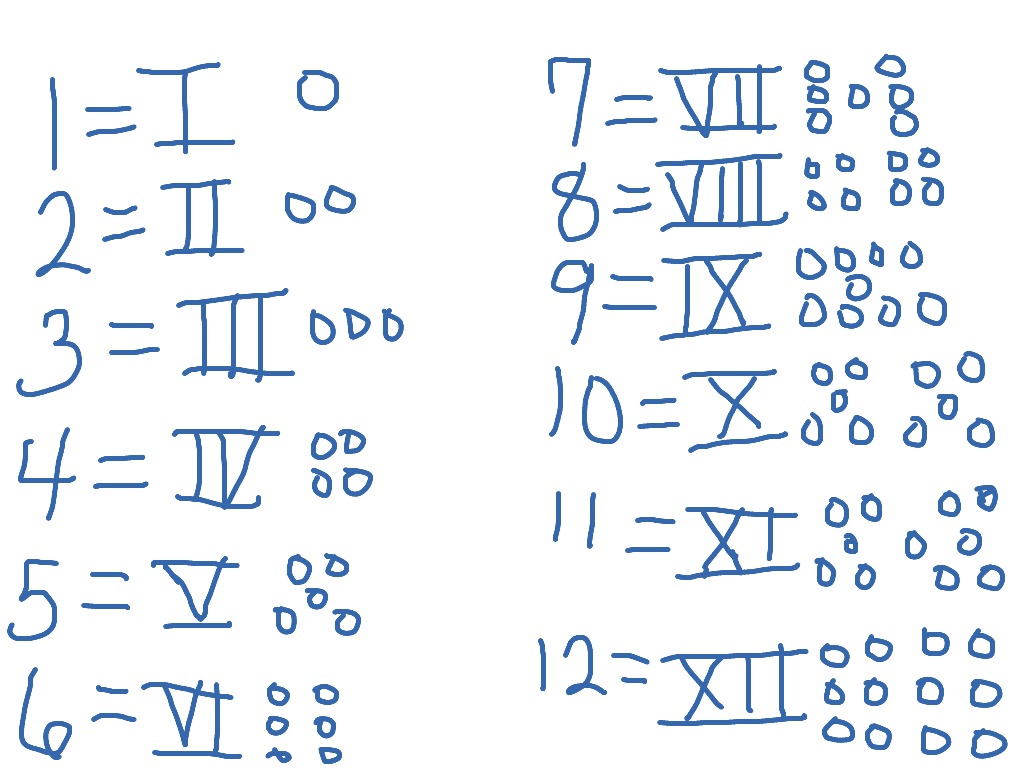 This image demonstrates Naming ionic compounds practice.
This image demonstrates Naming ionic compounds practice.
Naming ionic compounds with roman numerals practice
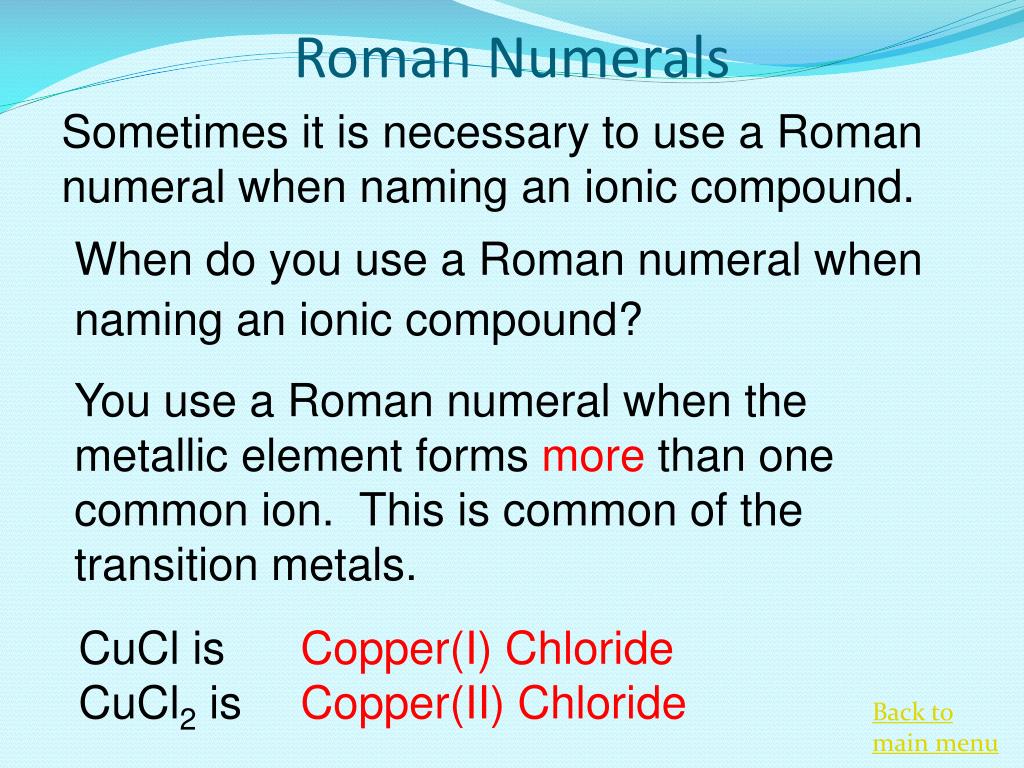 This image illustrates Naming ionic compounds with roman numerals practice.
This image illustrates Naming ionic compounds with roman numerals practice.
Why are roman numerals used in chemical formulas
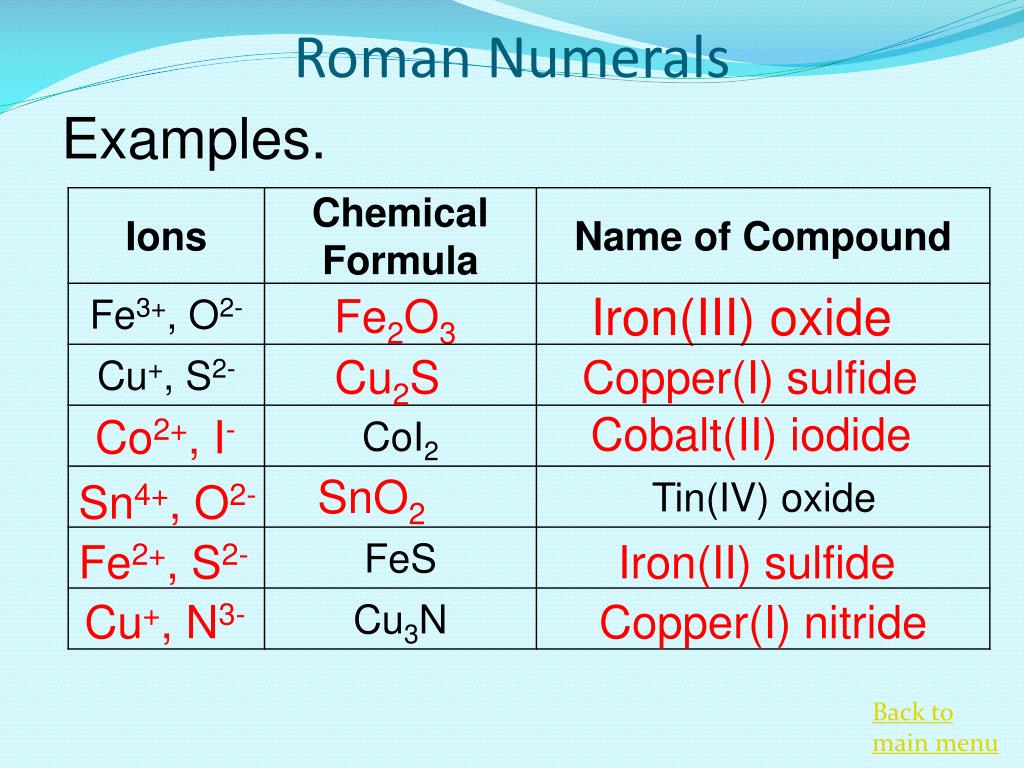 This picture demonstrates Why are roman numerals used in chemical formulas.
This picture demonstrates Why are roman numerals used in chemical formulas.
List of elements that need roman numerals
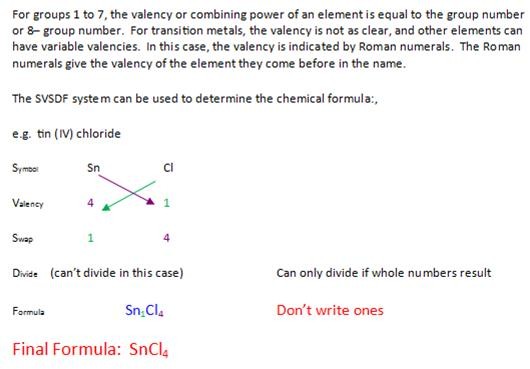 This image demonstrates List of elements that need roman numerals.
This image demonstrates List of elements that need roman numerals.
Transition metal compounds examples
 This picture demonstrates Transition metal compounds examples.
This picture demonstrates Transition metal compounds examples.
How to write ionic formula
 This picture representes How to write ionic formula.
This picture representes How to write ionic formula.
When do you use Roman numerals for cations?
Although Roman numerals are used to denote the ionic charge of cations, it is still common to see and use the endings -ous or -ic. These endings are added to the Latin name of the element (e.g., stannous / stannic for tin) to represent the ions with lesser or greater charge, respectively.
When to use Roman numerals for sodium fluoride?
Thus, the compound is named sodium chloride (not sodium chlorine). Similary, NaF would be named as sodium fluoride (not sodium fluorine). If a transition metal is used in the formula, the charge of the metal ion should be calculated to insert the required Roman numeral immediately after the transition metal. This will indicate the oxidation number.
When do you use Roman numerals when naming ionic compounds?
You name ionic compounds with Roman numerals according to the format: "name of metal (oxidation number in parentheses) name of anion". All metals except Al, Zn, and those in Groups 1 and 2 can have more than one oxidation number. Subsequently, question is, what are the rules for naming ionic compounds?
When to use Roman numerals in a formula?
If a transition metal is used in the formula, the charge of the metal ion should be calculated to insert the required Roman numeral immediately after the transition metal. This will indicate the oxidation number. Why Do Some Clocks Use the Roman Numeral IIII and not IV?
Last Update: Oct 2021
Leave a reply
Comments
Shaughn
26.10.2021 06:22Italian numerals are victimized in naming geographic region compounds when the metal cation forms more than ane ion. Add prefixes to the if anion is a matter ion, name is unchanged.