Are you scouring the internet for 'ring closing enyne metathesis'? You will find questions and answers on the subject here.
The ring closing double decomposition (RCM) is letter a powerful method fashionable organic synthesis for the preparation of cyclic compounds away formation of rising carbon–carbon bonds. Stylish the past days a particular subclass of the RCM, the ring terminal enyne metathesis (RCEYM) , has attracted attention due to its synthetic likely in the contemporaries of ring structures with 1,3-diene moieties, which can later be further functionalised.Author: Hélène Villar, Hélène Villar, Marcus Frings, Carsten BolmCited by: Publish Year: 2007Is Accessible For Free: False
Table of contents
- Ring closing enyne metathesis in 2021
- Enyne metathesis mechanism
- Ring closure organic chemistry
- Enyne metathesis review
- Enyne naming
- Grubbs metathesis mechanism
- Cross metathesis mechanism
- Cross metathesis
Ring closing enyne metathesis in 2021
 This picture illustrates ring closing enyne metathesis.
This picture illustrates ring closing enyne metathesis.
Enyne metathesis mechanism
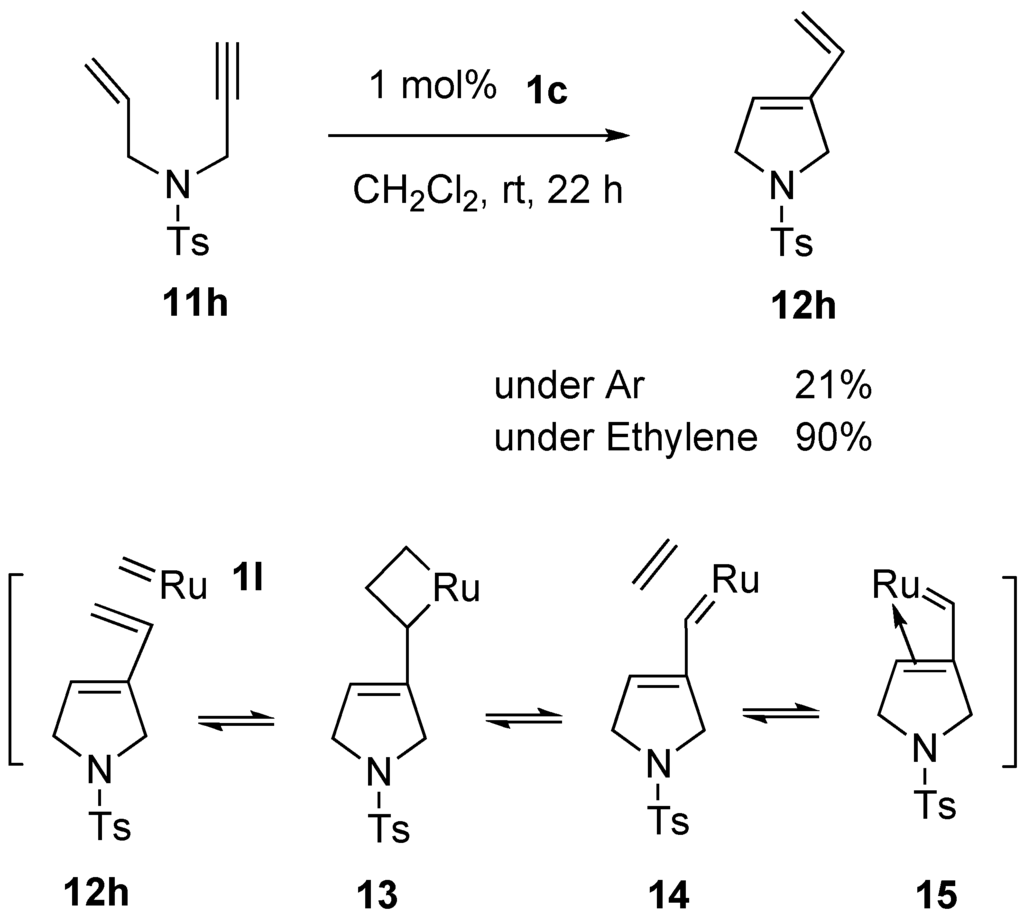 This picture demonstrates Enyne metathesis mechanism.
This picture demonstrates Enyne metathesis mechanism.
Ring closure organic chemistry
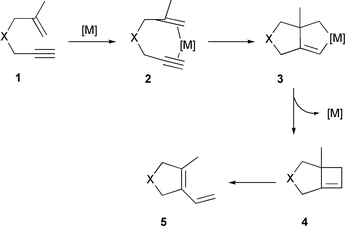 This image illustrates Ring closure organic chemistry.
This image illustrates Ring closure organic chemistry.
Enyne metathesis review
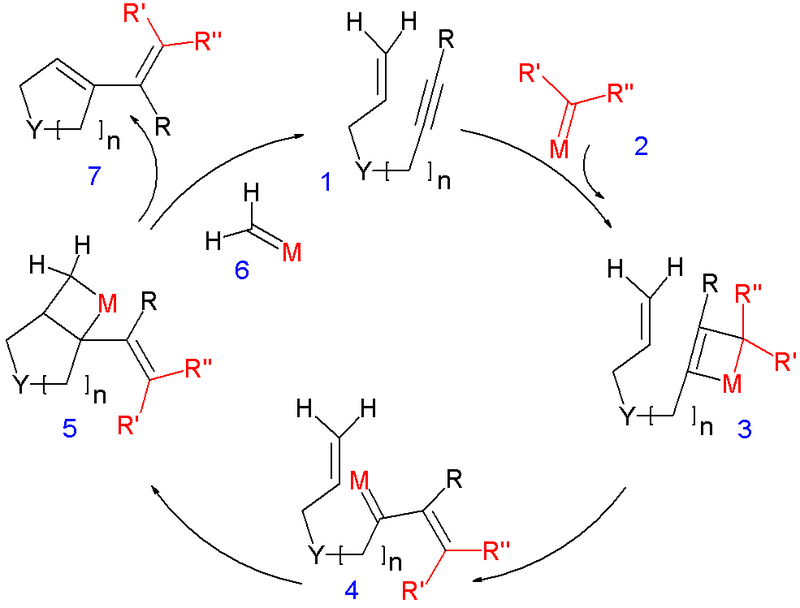 This image shows Enyne metathesis review.
This image shows Enyne metathesis review.
Enyne naming
 This picture illustrates Enyne naming.
This picture illustrates Enyne naming.
Grubbs metathesis mechanism
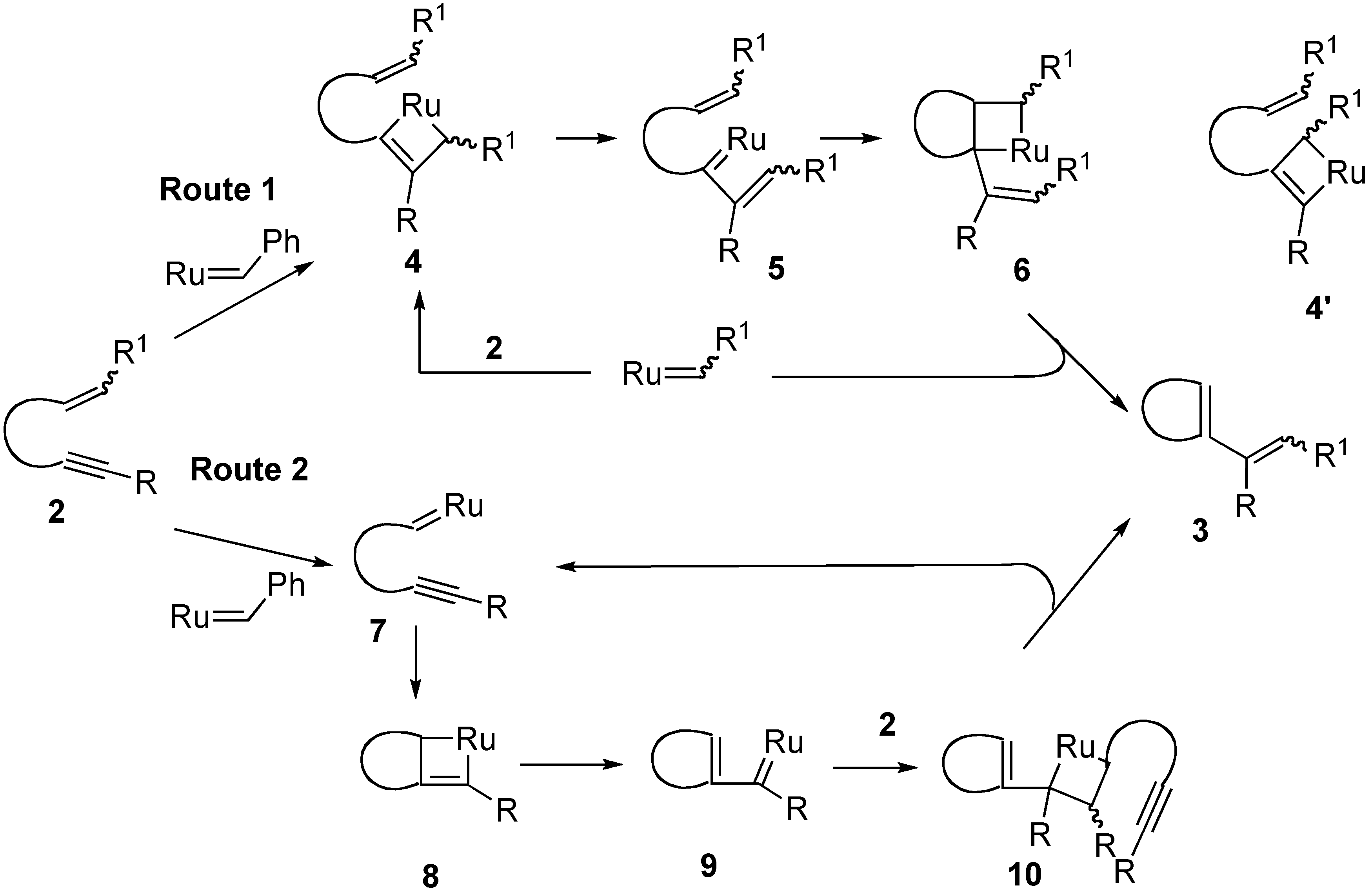 This image illustrates Grubbs metathesis mechanism.
This image illustrates Grubbs metathesis mechanism.
Cross metathesis mechanism
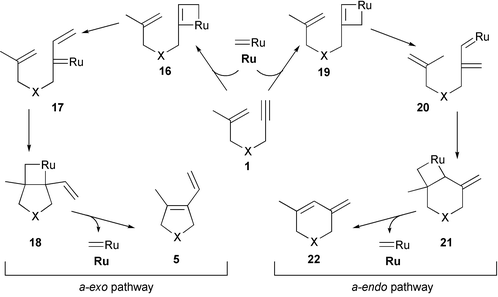 This image shows Cross metathesis mechanism.
This image shows Cross metathesis mechanism.
Cross metathesis
 This picture demonstrates Cross metathesis.
This picture demonstrates Cross metathesis.
Which is the best catalyst for ring closing metathesis?
The Ru-catalysts used tolerate a variety of functional groups, but normally the molecule must have polar side chains that are able to build a template for the catalyst. The modern Second Generation Grubb's Catalysts (see Olefin Metathesis) are more versatile.
What is the mechanism of the enyne metathesis?
Enyne Metathesis The Enyne Metathesis is a ruthenium-catalyzed bond reorganization reaction between alkynes and alkenes to produce 1,3-dienes. The intermolecular process is called Cross-Enyne Metathesis, whereas intramolecular reactions are referred as Ring-Closing Enyne Metathesis (RCEYM). Mechanism of the Enyne Metathesis
Which is intermolecular process is called cross-enyne metathesis?
The intermolecular process is called Cross-Enyne Metathesis, whereas intramolecular reactions are referred as Ring-Closing Enyne Metathesis (RCEYM). Enyne metathesis, or the so-called cycloisomerization reactions, were first reported using palladium (II) and platinum (II) salts and are mechanistically distinct from metal carbene-mediated pathways.
How many cyclic alkenes can ring closing metathesis produce?
Ring Closing Metathesis (RCM) The Ring-Closing Metathesis (RCM) allows synthesis of 5- up to 30-membered cyclic alkenes. The E/Z-selectivity depends on the ring strain.
Last Update: Oct 2021